Facilities
State-of-the-art clinical and bioanalytical infrastructure designed in line with global regulatory requirements
Synergen Bio’s dedicated clinical research facility in Pune is built to handle a wide spectrum of studies with precision, safety, and regulatory compliance. Our infrastructure supports both early-phase clinical studies, BA/BE studies and Phase 1 Clinical Trails with high-quality systems and real-time monitoring.

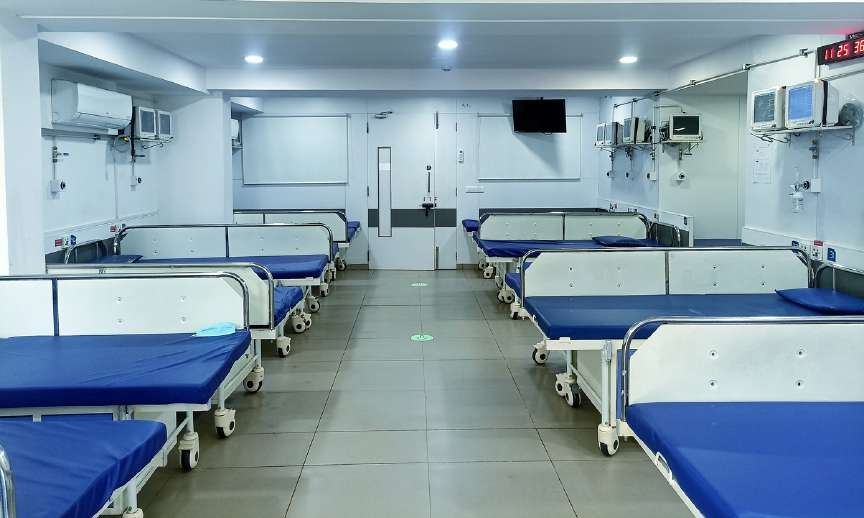
Clinical Facility:
Our clinical research unit is equipped with 160 beds (including a dedicated 16-bed Phase I unit) to support BA/BE and early-phase studies. The facility is fully GCP-compliant and designed to ensure subject safety, comfort, and strict regulatory adherence.
Key Facility Highlights:

- Full CCTV surveillance and access control systems for enhanced security
- Emergency nurse alert systems & synchronized clocks for time-sensitive procedures
- Six-bedded ICU with emergency backup
- Separate housing for male & female volunteers with dedicated changing & frisking rooms
- Informed consent area with provision for Audio and Video recording
- Volunteer registration & biometric system
- Dining and recreation areas
Sample Collection & Storage:
- Dedicated sample collection and processing areas
- Deep freezers at -70C and -20C with 24/7 monitoring and alarm systems
- Walk-in stability chambers for long-term sample integrity
- Controlled-access bioanalytical lab compliant with GLP standards

Bioanalytical Laboratory:
Our GLP-compliant bioanalytical lab is equipped with advanced LC-MS/MS instruments and supporting infrastructure for high-throughput and sensitive drug analysis.
- LC-MS/MS fleet: 2 system of Sciex 6500+, 9 systems of 4500
- Dedicated sample processing and storage areas
- -70°C, -30°C and -20°C deep
- Walk-in cooling chamber
- Unique DAS temperature monitoring system
- Sartorius analytical balances (Cubis & Secura)
Pharmacy:
Our in-house pharmacy ensures proper storage, dispensing, and accountability of investigational products. Sample storage is supported by robust backup and monitoring systems.
- Two walk-in stability chambers with alarm systems
- Laminar air flow for dispensing
- Controlled-access storage and accountability logs
- 24/7 power backup and monitoring
Archival Facility:
Our in-house archival facility ensures secure, long-term storage of essential study documents and data, fully compliant with GCP E6 R3 and ALCOA++ principles. Designed with advanced safety & environmental controls, it guarantees integrity, confidentiality & accessibility throughout the retention period.
- Fire-resistant compactor storage system
- Controlled temperature and humidity environment
- Restricted access area
- Secure handling and cataloging of study documents
- Long-term preservation as per regulatory requirements
Emergency & Safety Infrastructure:
Ensuring volunteer safety is our highest priority. Our clinical units are supported by advanced safety systems and trained staff
- Intensive care unit (ICU) with 6 beds, fully equipped for emergency support
- Tie up with full service multispeciality hospital located within 2kms of the facility
- OVIS software for tracking central volunteers’ cross participation
- VMS biometric identification data management software for internal registration of volunteers
